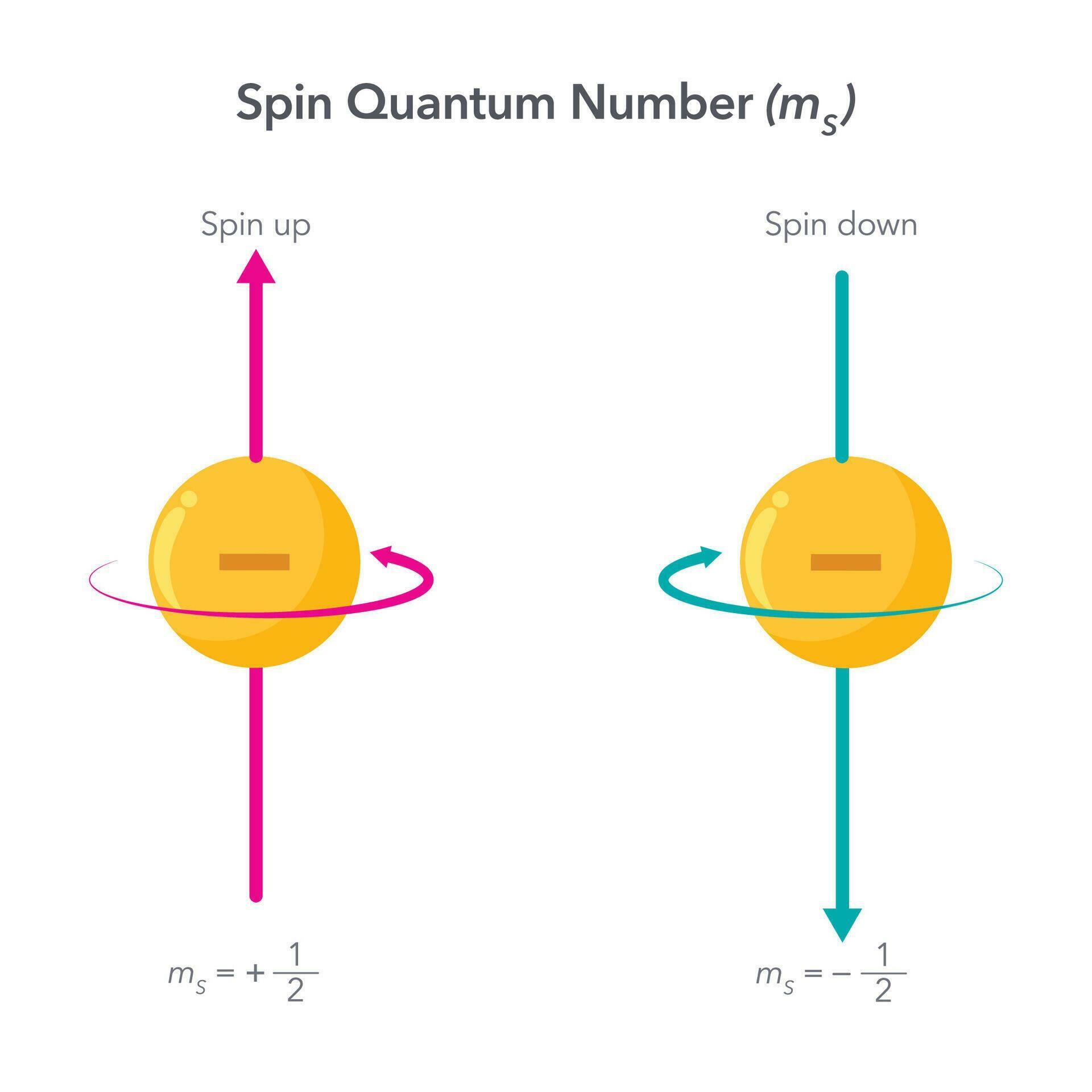
What Is An Spin Quantum Numbers . It is responsible for the electron having a magnetic north. electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. An electron spins around an. in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. in general, the spin quantum number is denoted with an s. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. For all electrons, however, s = ½. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. They represent the position, movement, and energy of the fundamental particle. spin quantum number (symbol: S) — this is the up/down or right/left spin of the electron. Denoted as ms m s, the electron spin is.
from www.vecteezy.com
electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. spin quantum number (symbol: It is responsible for the electron having a magnetic north. An electron spins around an. Denoted as ms m s, the electron spin is. in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. S) — this is the up/down or right/left spin of the electron. They represent the position, movement, and energy of the fundamental particle.
Spin Quantum Number physics vector illustration infographic 26586277
What Is An Spin Quantum Numbers electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. in general, the spin quantum number is denoted with an s. Denoted as ms m s, the electron spin is. It is responsible for the electron having a magnetic north. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. They represent the position, movement, and energy of the fundamental particle. S) — this is the up/down or right/left spin of the electron. An electron spins around an. For all electrons, however, s = ½. spin quantum number (symbol: electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation, free download What Is An Spin Quantum Numbers It is responsible for the electron having a magnetic north. S) — this is the up/down or right/left spin of the electron. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. in general, the spin quantum number is denoted with an s.. What Is An Spin Quantum Numbers.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Is An Spin Quantum Numbers S) — this is the up/down or right/left spin of the electron. in general, the spin quantum number is denoted with an s. It is responsible for the electron having a magnetic north. electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. An electron spins around an. For all electrons,. What Is An Spin Quantum Numbers.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is An Spin Quantum Numbers electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. They represent the position, movement, and energy of the fundamental particle. For all electrons, however, s = ½. It is responsible for the electron having a magnetic north. Denoted as ms m s, the electron spin is. quantum numbers are a. What Is An Spin Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers Activity PowerPoint Presentation, free download What Is An Spin Quantum Numbers in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. S) — this is the up/down or right/left spin of the electron. It is responsible for the electron having a magnetic north. quantum numbers are a set of numbers used to define the state in which a fundamental particle. What Is An Spin Quantum Numbers.
From www.slideserve.com
PPT Spin Quantum Number, m s PowerPoint Presentation, free download What Is An Spin Quantum Numbers in general, the spin quantum number is denoted with an s. in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. It is responsible for the electron having a magnetic north. An electron spins around an. They represent the position, movement, and energy of the fundamental particle. spin. What Is An Spin Quantum Numbers.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps What Is An Spin Quantum Numbers the spin quantum number (\(m_s\)) describes the angular momentum of an electron. electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. For all electrons, however, s = ½. S) — this is the up/down or right/left spin of the electron. They represent the position, movement, and energy of the fundamental. What Is An Spin Quantum Numbers.
From deepstash.com
Spin Quantum Numbers (ms) Deepstash What Is An Spin Quantum Numbers It is responsible for the electron having a magnetic north. They represent the position, movement, and energy of the fundamental particle. Denoted as ms m s, the electron spin is. in general, the spin quantum number is denoted with an s. S) — this is the up/down or right/left spin of the electron. spin quantum number (symbol: . What Is An Spin Quantum Numbers.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID834035 What Is An Spin Quantum Numbers spin quantum number (symbol: An electron spins around an. It is responsible for the electron having a magnetic north. in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. They represent the position, movement, and energy. What Is An Spin Quantum Numbers.
From www.slideserve.com
PPT Chapter 6 Electronic Structure of Atoms PowerPoint Presentation What Is An Spin Quantum Numbers in general, the spin quantum number is denoted with an s. They represent the position, movement, and energy of the fundamental particle. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. It is responsible for the electron having a magnetic north. . What Is An Spin Quantum Numbers.
From www.chemistrylearner.com
Physical Chemistry Page 2 of 3 Chemistry Learner What Is An Spin Quantum Numbers quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. An electron spins around an. in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. Denoted as ms m s, the electron spin is.. What Is An Spin Quantum Numbers.
From www.slideserve.com
PPT The fourth quantum number Spin PowerPoint Presentation, free What Is An Spin Quantum Numbers An electron spins around an. S) — this is the up/down or right/left spin of the electron. They represent the position, movement, and energy of the fundamental particle. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. Denoted as ms m s, the electron spin is. It is responsible for the electron having a magnetic north.. What Is An Spin Quantum Numbers.
From deepstash.com
Spin Quantum Numbers (ms) Deepstash What Is An Spin Quantum Numbers They represent the position, movement, and energy of the fundamental particle. electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. S) — this is the up/down or right/left spin of the electron. Denoted as ms m s, the electron spin is. in practice, spin is usually given as a dimensionless. What Is An Spin Quantum Numbers.
From ar.inspiredpencil.com
Spin Quantum Numbers What Is An Spin Quantum Numbers in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. Denoted as ms m s, the electron spin is. S) — this is the up/down or right/left spin of the electron. They represent the position, movement, and energy of the fundamental particle. quantum numbers are a set of numbers. What Is An Spin Quantum Numbers.
From www.youtube.com
Class 11 chap 2 Spin Quantum Number Quantum Numbers JEE / NEET What Is An Spin Quantum Numbers in general, the spin quantum number is denoted with an s. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. For all electrons, however, s = ½. spin. What Is An Spin Quantum Numbers.
From slideplayer.com
Chapter 5 Quantum Numbers Part ppt download What Is An Spin Quantum Numbers the spin quantum number (\(m_s\)) describes the angular momentum of an electron. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. spin quantum. What Is An Spin Quantum Numbers.
From education-portal.com
Spin Quantum Number Definition & Example Video & Lesson Transcript What Is An Spin Quantum Numbers in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. An electron spins around an. For all electrons, however, s = ½. Denoted as. What Is An Spin Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers and Electronic Configuration PowerPoint What Is An Spin Quantum Numbers For all electrons, however, s = ½. An electron spins around an. the spin quantum number (\(m_s\)) describes the angular momentum of an electron. in practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by. quantum numbers are a set of numbers used to define the state in which. What Is An Spin Quantum Numbers.
From www.slideserve.com
PPT CHAPTER 6 PowerPoint Presentation, free download ID810763 What Is An Spin Quantum Numbers An electron spins around an. electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. It is responsible for the electron having a magnetic north. quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. . What Is An Spin Quantum Numbers.